Digital Transformation solutions for Pharmaceutical Manufacturing
Why do we need to adopt this digital process
Digital Transformation solutions for Pharmaceutical Manufacturing
Many instrument logs like calibration report, logbooks for temperature monitoring, experiments logs etc are still in paper-based records. Having such a fast growth in mobile applications development, the companies should adopt to digital way of recording this data entry process, which helps in many ways.
Digital Transformation solutions for Pharmaceutical Manufacturing
Advantages of Electronic logbook
- Simplify processes related to administration and use of validation documents, logbooks and forms
- Create forms and check lists with workflows for registration of GxP activities supporting batch release.
- It is very easy to track the GMP records during external audits
- 100% reduction of paper and manual data entries, which comes with, automated system.
- Records cannot be tampered or misplaced. Ensures data integrity
- Real time data entry from assembly line.
- Quick review and approval process.
- Integration with QR code for traceability solutions
- The solution will improve the quality of data for analytics and reporting and support the instant release of product.
- Enable digitalisation such as analytics and Robotic Process Automation
Digital Transformation using Mobility – Having android-based tablets at shop floor level.
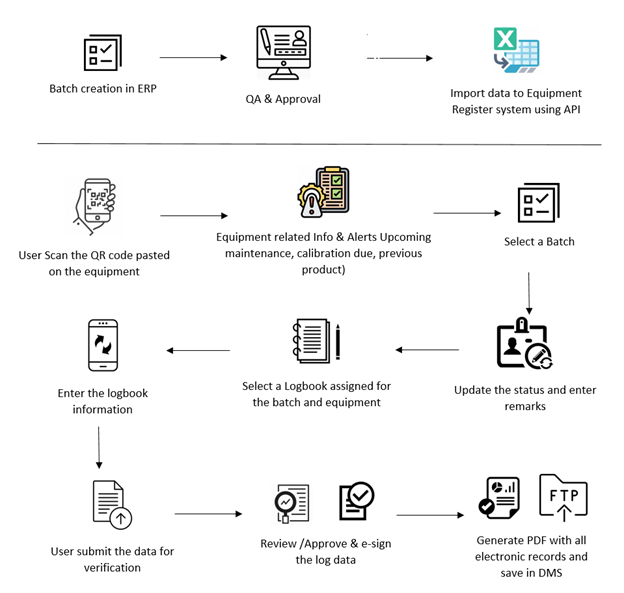
- Batch creation for a product is executed in ERP which will have the following (Batch code, Product, Quantity, Equipment Batch Status)
- Each equipment in the process flow is QR coded with equipment information like IQ date , next calibration date etc
- The operator scans equipment QR code using mobile application. The mobile apps open the logbook that needs to be updated.
User does the following in the mobile apps
- Scan the equipment /select the equipment
- System shows the status of equipment with previous batch information.
- Update the status with remarks (In use, To be cleaned, cleaning, Ready for use, Maintenance activity)
- Perform the activity and update the logbook.
Each entry will have a transaction id with approval flow. After the publishing
Equipment Dashboard
- Web based application to have equipment wise status view
- Shop floor will have thin client based system with a TV or Display unit. This display unit will show the equipment dashboard
Security Challenges – Mobile device management approach
- To have an android based application at end user level will have lot of security challenges.
- To solve this problem, we can adopt mobile-based device management tools.
- Microsoft Intune helps to solve the security-based issues in an enterprise network. This also adopts all internal IT security policy and ensure the data security
You May also View Other Related Articles
Smarter Approach To Implement Paperless Validation Solution For Your Organization
Going for paperless validation and implementing a comprehensive Validation Management System (VLMS) requires a change in mindset in the leadership ...
How GoVal can help the organization to go for digital validation and get rid of manual processes?
Manual To Automation More and more organization is adopting to software based validation solution to make their processes paperless, less ...
Talk to us
Find out how Goval can make your validation more efficient and smarter.
Start your digital validation by speaking to our experts.
